The basic condition for medical equipment to continuously improve the level of medical science and technology is also an important sign of modernization. Medical equipment has become an important field of modern medicine. The development of medical care depends to a great extent on the development of instruments, and even in the development of the medical industry, its breakthrough bottleneck has played a decisive role.
Medical equipment refers to instruments, equipment, utensils, materials or other items used alone or in combination on the human body, and also includes required software. The therapeutic effect on the body surface and the body is not obtained by means of pharmacology, immunology or metabolism, but medical device products play a certain auxiliary role. During the period of use, it aims to achieve the following intended purposes: prevention, diagnosis, treatment, monitoring, and relief of disease; diagnosis, treatment, monitoring, relief, and compensation of injury or disability; research, substitution, and adjustment of anatomical or physiological processes ; Pregnancy control.
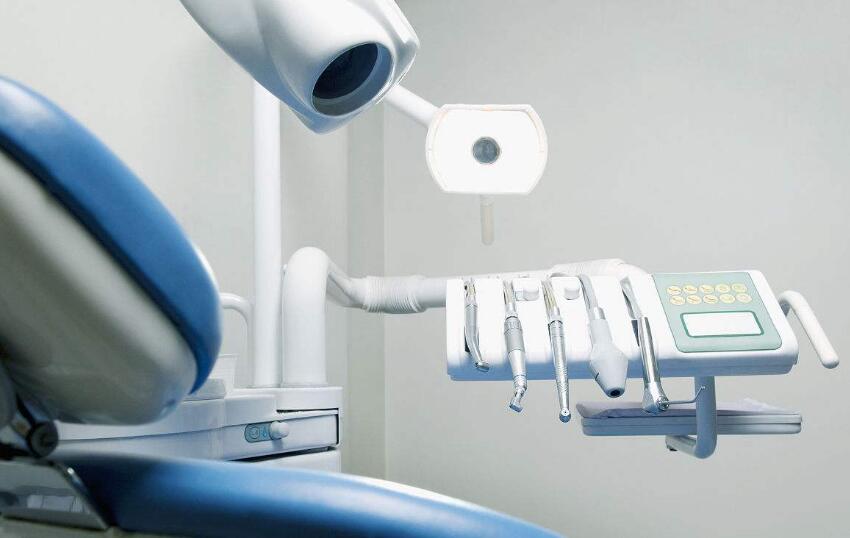
Medical equipment in a broad sense includes medical equipment and household medical equipment, while professional medical equipment does not include household medical equipment and equipment. It can be seen that they are closely connected at the same time, but also an inclusive relationship, and the subtle differences are not difficult to see.
The repair and maintenance of large medical equipment, the installation of equipment and the scrapping of equipment are all one of the main tasks of the equipment department in the hospital, which is directly related to the safety of the use of instruments, the effectiveness of the inspection and testing of clinical medical equipment, The collaboration and continuity of medical work in the entire hospital. The basic point of the development and design of the system is how the equipment department uses limited manpower, material resources and limited resources to ensure the normal utilization rate of the equipment in cost-effective consideration to achieve a higher degree of autonomous maintenance is a very important Subject.
Intelligent
A large hospital equipment maintenance management system should not simply repeat manual procedures, it should be a program with intelligent features. This system contains several EOQ modules, especially set the equipment maintenance alarm prompt. When a piece of equipment to be repaired is sent to the Equipment Section for maintenance, the computer will automatically remind (according to the validity period of the maintenance equipment) that the maintenance engineer has not repaired in time, and the alarm will be divided into three levels (and audible and visual alarm). Normally when the system is in the login interface, it adopts the server module type, and it is checked every once in a while. If the maintenance equipment is to be repaired, the system will promptly remind the engineer to carry out maintenance with sound and light alarms on the operation interface.
The system provides modules such as equipment classification, equipment management, spare parts management, information management, report output and statistical analysis. It can perform statistical analysis on various maintenance work indicators and display the statistical analysis results in the form of a table. Such as the maintenance statistics of the maintenance equipment, the number of equipments sent for inspection, the repair rate of the equipment maintenance, the repair rate of the equipment maintenance and the inventory of the components, and the analysis of the equipment scrap factor.
For data integrity and security, we use code to implement automatic database backup and restore functions, and system administrators can also manually backup and restore data. Another technique is a strict authorization mechanism. The administrator assigns different management rights according to the responsibilities of the engineer operator.
stability
The computer system uses Windows XP Advanced Server. Through background data collection, it provides engineers with a high-performance client and server platform. The data processing method using this system is quite concise. It can process various display formats and print data reports. Complex data and reports can be processed freely.

There is no power failure in the hospital. Once the power failure occurs, it may threaten the patient's life. Similarly, medical equipment cannot be powered off, so medical equipment has very strict requirements for power supply specifications. Therefore, there are special specifications in the design of medical device batteries.
China's medical electronic product demand growth is higher than the global average-huge population base and the rapidly increasing aging population and people's increasing health awareness, driven by national policies, medical informatization and technological revolution. China's medical electronic product market demand continues to maintain rapid growth.
In addition, China has begun to implement the 12th Five-Year Plan, in which the future development of medical equipment has the following three goals: 1) accelerate the development of the domestic medical equipment industry; 2) implement a unified procurement system; 3) domestic medical institutions Should prefer to buy domestic medical equipment. With the gradual implementation of this medical reform plan, domestic medical equipment manufacturers have made all preparations to take advantage of this rare development opportunity to fully develop a new generation of medical equipment.
In addition to the medical equipment must comply with internationally recognized standards, other basic performance and whether the power supply meets the specifications are very important, because the patient's health will directly or indirectly accept its impact. Any electronic medical equipment related to patient care, clinical treatment, health monitoring, or image scanning, if the power supply fails to be powered, loses power or other problems, the consequences will directly affect the health of the patient, even to the patient. Causes temporary or permanent injury.
Taking laboratory instruments or diagnostic equipment as an example, once the power supply fails, medical staff cannot make a correct diagnosis in time, and sometimes it is necessary to do more tests, which wastes time and increases the spirit of medical staff and patients. burden.
In addition, once the power supply of a medical device fails, even if it does not immediately pose a security problem, it will not be able to perform its basic functions. Therefore, medical device developers must not only plug design loopholes during the product design stage, but also throughout the product life cycle The relevant risks are continuously managed in order to avoid equipment failure.

During its lifetime, medical device circuits will suffer from various electrical threats in many ways. In short, any power supply or communication interface is a potential entry point for electrical transients, and it is easily damaged during the life of the device. Therefore, pay attention to the power supply (battery pack, DC input, AC input), microprocessor, microphone / speaker line, communication interface (wired and wireless), sensor, LCD display, keypad and keys.
For more high-end equipment (imaging, diagnostics, and laboratory equipment), due to its more complex and more electrical threats, additional protection devices are required. Circuits such as AC power supplies and high-voltage DC power supplies require surge protection solutions that can handle much higher energy than portable devices.
Overvoltage suppression equipment (device):Gas discharge tubes (GDTs) are usually used to protect telecommunication lines, data communication lines, and signal lines of other devices (equipment) from surge voltage. The device can withstand surge currents up to 40,000 amps, making it ideal for reducing transients caused by lightning.
Varistors (variable resistors) allow currents generated by excessively high transient voltages to avoid sensitive equipment. There are two main types:
Multilayer varistors (MLVs) can provide protection for medium and low energy transients (0.05-2.5 Joules) for sensitive equipment powered by 0-120V DC. These multi-layer varistors are most commonly used for ESD protection.
• Metal oxide varistors (MOVs) can provide a rated energy of 0.1-10,000 joules, allowing sensitive components to avoid transient currents. For low-voltage DC power ports or signal ports, the varistor combines the advantages of high surge and small size discs, providing an ideal choice for space-saving designs. For example, a 10mm varistor can withstand a maximum surge current of 2,000A, which is four times the maximum surge current of a standard MOV of the same size. The device protects circuits from electrical threats such as induced lightning strike interference, system switching transient pulses, and abnormally fast transients in power supplies.
Polymer ESD suppressors have low junction capacitance (~ 0.05pF) and fast clamping capability, so they are ideal for high-speed digital I / O and RF lines. Low junction capacitance helps ensure that no bit errors or distortions will occur.
Transient voltage suppression (TVS) diodes protect various circuits and components from the threats common to DC power lines. The pn junction cross-section of this diode is much larger than that of conventional diodes, so that a large current can be conducted to ground without damage, so that the transient voltage is suppressed to a level lower than that of other technologies. The transient power rating is between 400 watts and 15,000 watts, with a maximum waveform of 15,000A @ 8x20us.
The semiconductor diode protection array (SPA) is designed to protect analog and digital signal lines from ESD and other transient overvoltages. In addition, a multi-channel array can be used to provide ESD protection in a smaller space, and the clamping voltage is lower than other technologies, thereby providing the best protection possible.
Semiconductor discharge tube design applications suppress transient overvoltages in telecommunications and data communications equipment, and can conduct currents as high as 5,000A to ground within a few nanoseconds of reaching breakdown voltage.
Overcurrent protection device:Fuses are the most commonly used overcurrent protection devices, which are divided into fast-break type and slow-break type (delay) type. The second type can minimize the number of repeated replacements when there is a short-lived but repetitive overcurrent "pulse". In portable applications, small surface mount fuses are often used to save space and block over-voltage and short-circuit currents.
PTC thermistors are repeatable devices that replace fuses. When the current increases, increase the PTC resistance and automatically limit the current. Generally, the use of polymer (PPTC) materials can form a clear inflection point between impedance and temperature. Once the overload disappears, the PPTC will cool down and restore the circuit to normal operation. Therefore, there is no need to replace the fuse.

The first thing to consider in the design of medical electronic products is the requirements of risk management. For example, in addition to meeting the basic risk management requirements of the ISO14971 standard, it must also meet the requirements of standards such as IEC60601-1 Ed.3 and IEC62304. "In the actual risk management operation, equipment manufacturers should also pay attention to software risk management, post-market risk management, etc. In addition, during the design process, manufacturers should pay attention to the data collection of similar products, especially when designing some high-risk products, this It is very useful for equipment manufacturers, because if we can do a detailed analysis of the collected information, we can effectively avoid the mistakes that have been made by previous people and improve the design reliability. "Zhou Saixin said.
The tools currently used in risk management are commonly used in process failure analysis (PFMEA) and design failure analysis (DFMEA). PFMEA is the failure analysis of the entire production process when the product is transferred from design to production; DEFEA is the analysis of the failure mode effect during the design process. Generally, these two analysis techniques are often used in products with high risk and high reliability requirements. In the analysis of the impact of failure modes, the level of risk must also be judged from the three points of severity, probability and detectability.
"There are two key points in the design of good risk control. One is to follow the trilogy principle of inherent safety, protective measures, and safety information; the second is to do a good job of patient safety under a single failure." Zhou Saixin pointed out. Specifically, the trilogy of inherent safety, protective measures, and safety information is: if risks are identified, the first consideration is how to achieve inherent design safety; when inherent safety cannot be achieved, protective measures and safety information should be marked . For example, when designing a product, the first consideration is how to make it without hazardous voltage, rather than how to deal with it after hazardous voltage; when hazardous voltage is unavoidable, you should take protective measures such as insulation and paste it where hazardous voltage may occur Put a warning label to remind users.
reliabilityThe importance of reliability to medical electronics is self-evident. Due to the increasingly complex use environment, the reliability challenges facing products are also increasing. For example, the previous equipment may only be used at the bedside. However, in-hospital transfers and out-of-hospital emergency situations frequently occur. When moving medical equipment, accidents such as falls and collisions may occur. In addition, the use of equipment is becoming heavier, and many equipment is often used. It is not shut down for 24 hours or even for several months. Under these circumstances, how to ensure the reliability of the equipment is a problem that equipment manufacturers have to think about.
The technological upgrading of the electronics industry has also made equipment manufacturers face new challenges. The miniaturization of electronic devices, high-density packaging, and high-density BGA / QFN soldering under lead-free processes are an important development trend. It has become the mainstream in consumer electronic products such as mobile phones and tablet computers. Medical electronics is different from consumer electronics. It has its own unique industrial chain. At present, the packaging that matches the capabilities of the industrial chain is still 0402 / 0.5mm packaging process. At this level, the SMT patch of most products can guarantee a certain yield. And reliability, but medical electronic equipment manufacturers will always face the problem of product reliability brought by miniaturized packaging. In addition, the continuous advancement of semiconductor technology has brought about rapid chip replacement, and the reliability problems caused by chip replacement are inevitable. "When using a new chip, you need to pay attention to the early defects of the chip. Nowadays, the complexity of the chip is getting higher and higher. Don't think that the reliability of the chip will be guaranteed. Like software, it will have some early immature factors. Using new chips can enjoy the new technological advancements it brings, but it also faces certain risks. "Zhou Saixin said.
Software reliability in product reliability design is also critical. Now that the product is becoming more and more software-based, it is possible that 70% or 80% of the energy is spent on software design. Zhou Saixin pointed out that software reliability design should pay attention to two points: the use of OTS software / SOUP software and the choice of operating system. OTS software / SOUP software are all software from unknown sources. Some equipment manufacturers may download some driver software in the open source community. At this time, special attention should be paid because these software are not necessarily developed in accordance with the requirements of medical devices. Reliability is difficult to guarantee. In addition, the choice of operating system is also directly related to the reliability of the product. At present, there are many operating systems, open source and closed, and each has its own focus on real-time and GUI. As for which operating system to choose, it is mainly from the company ’s products and development. Starting from capabilities, choose an operating system that matches the development capabilities.
Other key factorsThe design of medical electronic products needs to meet the requirements of safety regulations. At present, the IEC60601-1 standard is a safety standard widely used in the world and suitable for medical equipment. Since June this year, Europe has enforced the third edition of the safety standards, which applies to all new and old products. In addition to proposing risk management for safety and basic performance, the third edition of the standard also proposes two different concepts for operator protection measures (MOOP) and patient protection measures (MOPP), including bottom openings, Impact testing, altitude and pollution considerations, etc. For new requirements in electrical safety and mechanical safety, equipment manufacturers should ensure that they meet the new requirements in safety regulations when developing products.
From the perspective of the usability design of medical products, the main usability problems faced by the current products include false alarms, complex setup and maintenance, and tender-oriented function accumulation. These will greatly reduce the usability of the products. Zhou Saixin believes that the solution to the problem is intelligent design, such as adding an intelligent alarm engine, intelligent lead switching, and intelligent working state recognition. In addition, new technologies for human-computer interaction can be added, including language prompts, new display technologies, and touch UI applications, to increase the ease of use of the device. He said: "Consumer electronics products in the new environment have a great impact on doctors' habits, especially handheld devices and digital products. Doctors also hope that the equipment used in daily work can have similar characteristics."
Driven by Internet technology, the use of wireless technology to interconnect devices has become an important development trend for medical electronic products. WiFi, LAN, Bluetooth, ZigBee, 3G, etc. are common wireless technologies. Different wireless technologies have different characteristics and different scopes of application. "I personally prefer Bluetooth 4.0 technology, which can make the power consumption very low to meet the current product low-power design requirements." Zhou Saixin said. He pointed out that wireless design should also consider many factors including frequency band selection, wireless authentication, data roaming, device positioning, pairing, access speed and so on.
Ningbo Autrends International Trade Co.,Ltd. , https://www.vapee-cigarettes.com